Abstract
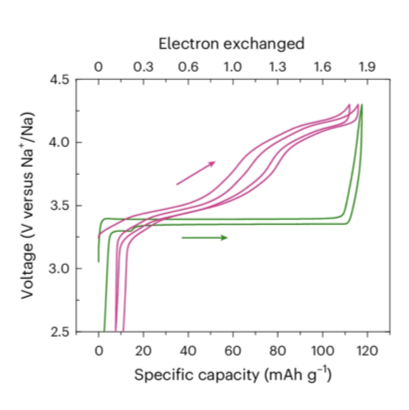
We report on single-phase NaxV2(PO4)3 compositions (1.5 ⋜ x ⋜ 2.5) of the Na super ionic conductor type, obtained from a straightforward synthesis route. Typically, chemically prepared c-Na2V2(PO4)3, obtained by annealing an equimolar mixture of Na3V2(PO4)3 and NaV2(PO4)3 , exhibits a specific sodium-ion distribution (occupancy of the Na(1) site of only 0.66(4)), whereas that of the electrochemically obtained e-Na2V2(PO4)3 (from Na3V2(PO4)3) is close to 1. Unlike conventional Na3V2(PO4)3 , when used as positive electrode materials in Na-ion batteries, the NaxV2(PO4)3 compositions lead to unusual single-phase Na+ extraction/insertion mechanisms with continuous voltage changes upon Na+ extraction/insertion. We demonstrate that the average equilibrium operating voltage observed upon Na+ deintercalation from single-phase Na2V2(PO4)3 is increased up to an average value of ~3.70 V versus Na+/Na (thanks to the activation of the V4+/V5+ redox couple) compared to 3.37 V versus Na+/Na in conventional Na3V2(PO4)3, thus leading to an increase in the theoretical energy density from 396.3 Wh kg-1 to 458.1 Wh kg-1. Electrochemical and chemical Na+ deintercalation from c-Na2V2(PO4)3 enables complete Na-ion extraction, increasing energy density.