Abstract
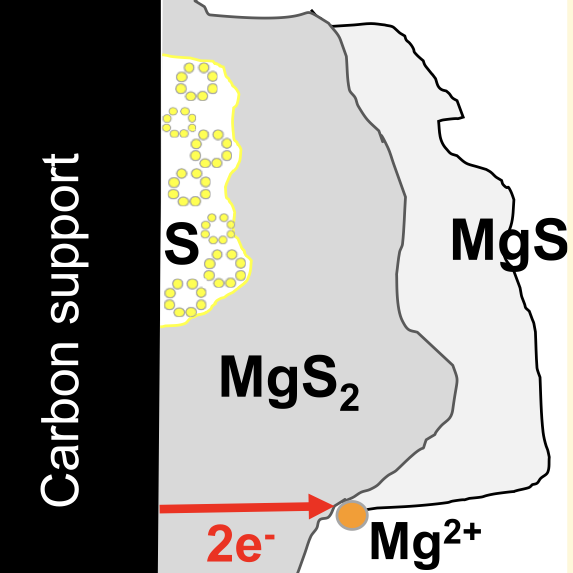
Rechargeable Mg−S batteries are attractive for next-generation energy storage devices due to their high theoretical energy density (1684 Wh kg−1 and 3286 Wh l−1) and low costs. The poor cycling performance of Mg−S batteries is linked to the formation of the solid discharge products, i.e., MgS2 and MgS, which are electronic and ionic insulators. However, the formation of MgS itself contradicts such a premise because it requires further oxidation of MgS2. Indeed, the insulating nature of MgS2 should inhibit such an oxidation process in the first place. Using first-principles calculations and ab initio molecular dynamics simulations, we evaluate the charge transport associated with point defects in MgS2 and MgS. In MgS2, the single-electron polaron is the most abundant type of defect that emerges from our model, which appears at a low concentration at thermodynamic equilibrium but displays high mobility. However, under conditions far from thermodynamic equilibrium, mimicking those for battery operations, the concentration of electron polarons increases, enhancing the electronic conductivity in MgS2. We demonstrate that in regimes far from thermodynamic equilibrium, the single-electron polarons coalesce to form double-electron polarons, whose mobilities are similar to that of a single-electron polaron. MgS2 holds electronic conduction through a polaron migration mechanism for ≤3 μm thick deposits, enabling further oxidation to form MgS. For MgS, our model suggests that the doubly positive Mg interstitial and doubly negative Mg vacancy are identified as the prevalent defects with high concentrations. However, due to the low mobility of these defects, their contribution to charge transport is negligible, which stops the oxidation process and severely hinders battery cyclability. Our results indicate that rechargeable Mg−S batteries can be developed if we ensure that the battery discharge does not push the oxidation process beyond the formation of MgS2.