Abstract
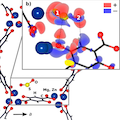
In situ infrared spectroscopy and ab initio density functional theory (DFT) calculations are combined to study the interaction of the corrosive gases SO2 and NO2 with metal organic frameworks M-MOF-74 (M = Zn, Mg, Ni, Co). We find that NO2 dissociatively adsorbs into MOF-74 compounds, forming NO and NO3–. The mechanism is unraveled by considering the Zn-MOF-74 system, for which DFT calculations show that a strong NO2–Zn bonding interaction induces a significant weakening of the N–O bond, facilitating the decomposition of the NO2 molecules. In contrast, SO2 is only molecularly adsorbed into MOF-74 with high binding energy (>90 kJ/mol for Mg-MOF-74 and >70 for Zn-MOF-74). This work gives insight into poisoning issues by minor components of flue gases in metal organic frameworks materials.