Abstract
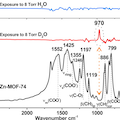
Water dissociation represents one of the most important reactions in catalysis, essential to the surface and nano sciences [e.g., Hass et al., Science, 1998, 282, 265–268; Brown et al., Science, 2001, 294, 67–69; Bikondoa et al., Nature, 2005, 5, 189–192]. However, the dissociation mechanism on most oxide surfaces is not well understood due to the experimental challenges of preparing surface structures and characterizing reaction pathways. To remedy this problem, we propose the metal organic framework MOF-74 as an ideal model system to study water reactions. Its crystalline structure is well characterized; the metal oxide node mimics surfaces with exposed cations; and it degrades in water. Combining in situ IR spectroscopy and first-principles calculations, we explored the MOF-74/water interaction as a function of vapor pressure and temperature. Here, we show that, while adsorption is reversible below the water condensation pressure (∼19.7 Torr) at room temperature, a reaction takes place at ∼150 °C even at low water vapor pressures. This important finding is unambiguously demonstrated by a clear spectroscopic signature of the direct reaction using D2O, which is not present using H2O due to strong phonon coupling. Specifically, a sharp absorption band appears at 970 cm–1 when D2O is introduced at above 150 °C, which we attribute to an O–D bending vibration on the phenolate linker. Although H2O undergoes a similar dissociation reaction, the corresponding O–H mode is too strongly coupled to MOF vibrations to detect. In contrast, the O–D mode falls in the phonon gap of the MOF and remains localized. First-principles calculations not only positively identify the O–D mode at 970 cm–1 but derive a pathway and kinetic barrier for the reaction and the final configuration: the D (H) atom is transferred to the oxygen of the linker phenolate group, producing the notable O–D absorption band at 970 cm–1, while the OD (or OH) binds to the open metal sites. This finding explains water dissociation in this case and provides insight into the long-lasting question of MOF-74 degradation. Overall, it adds to the understanding of molecular water interaction with cation-exposed surfaces to enable development of more efficient catalysts for water dissociation.