Abstract
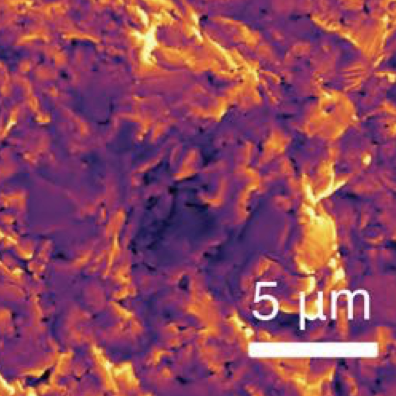
The growing demand for energy storage systems sparks a race to build inexpensive and safer rechargeable batteries. All-solid-state sodium (Na)-ion batteries are a competitive alternative to their lithium (Li) analogs due to the lower cost of Na resources. The Na SuperIonic CONductors Na1+xZr2SixP3-xO12 0 ≤ x ≤ 3 (NZSP) are widely studied as solid electrolytes. However, synthesized NZSPs always contain m-ZrO2 as the main impurity phase, which may lead to a lower Na-ion ionic conductivity within the solid-electrolyte layer. Here, we synthesize zirconia-free NZSP by engineering the quantity of Zirconium (Zr) precursors. Synchrotron X-Ray diffraction, Raman spectroscopy, and density functional theory simulations reveal zirconia-free NZSP. Impedance spectroscopy measurement of zirconia-free NZSP reveals an impressive total ionic conductivity of ~3.49 mS cm–1 with a bulk conductivity of ~10.05 mS cm–1 at room temperature, making it an excellent Na-ion conductor for all-solid-state batteries. These results pave the way towards the synthesis optimization of impurity-free complex solid-electrolytes, which are important if solid-state batteries are to be commercialized.