Abstract
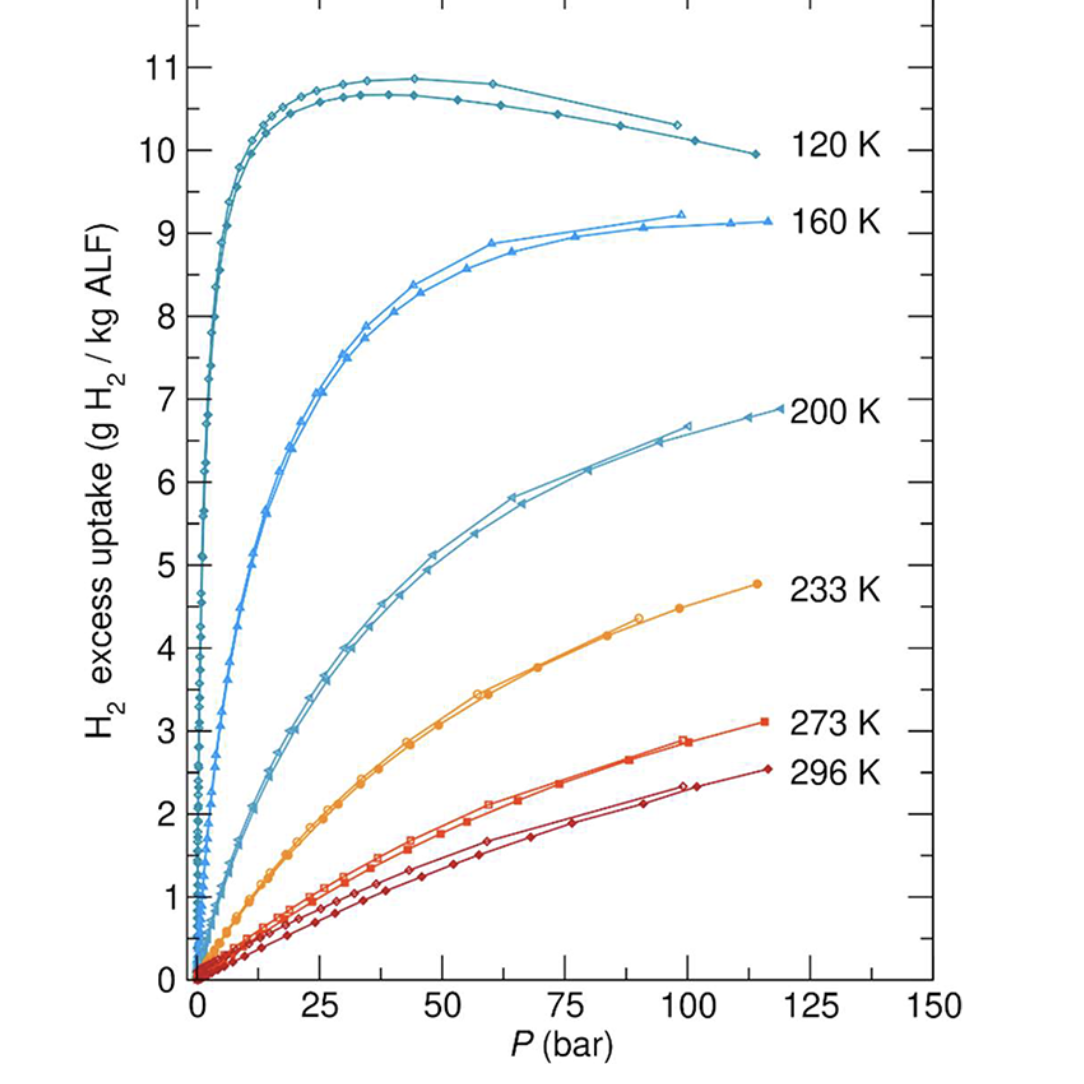
Long-duration storage of hydrogen is necessary for coupling renewable H2 with stationary fuel cell power applications. In this work, aluminum formate (ALF), which adopts the ReO3-type structure, is shown to have remarkable H2 storage performance at non-cryogenic (>120 K) temperatures and low pressures. The most promising performance of ALF is found between 120 K and 160 K and at 10 bar to 20 bar. The study illustrates H2 adsorption performance of ALF over the 77 K to 296 K temperature range using gas isotherms, in situ neutron powder diffraction, and DFT calculations, as well as technoeconomic analysis (TEA), illustrating ALF\’s competitive performance for long-duration storage versus compressed hydrogen and leading metal\–organic frameworks. In the TEA, it is shown that ALF\’s storage capacity, when combined with a temperature/pressure swing process, has advantages versus compressed H2 at a fraction of the pressure (15 bar versus 350 bar). Given ALF\’s performance in the 10 bar to 20 bar regime under moderate cooling, it is particularly promising for use in safe storage systems serving fuel cells.