Abstract
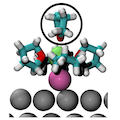
Multivalent (MV) battery architectures based on pairing a Mg metal anode with a high-voltage (∼3 V) intercalation cathode offer a realistic design pathway toward significantly surpassing the energy storage performance of traditional Li-ion-based batteries, but there are currently only few electrolyte systems that support reversible Mg deposition. Using both static first-principles calculations and ab initio molecular dynamics, we perform a comprehensive adsorption study of several salt and solvent species at the interface of Mg metal with an electrolyte of Mg2+ and Cl- dissolved in liquid tetrahydrofuran (THF). Our findings not only provide a picture of the stable species at the interface but also explain how this system can support reversible Mg deposition, and as such, we provide insights in how to design other electrolytes for Mg plating and stripping. The active depositing species are identified to be (MgCl)+ monomers coordinated by THF, which exhibit preferential adsorption on Mg compared to possible passivating species (such as THF solvent or neutral MgCl2 complexes). Upon deposition, the energy to desolvate these adsorbed complexes and facilitate charge transfer is shown to be small (∼61–46.2 kJ mol–1 to remove three THF from the strongest adsorbing complex), and the stable orientations of the adsorbed but desolvated (MgCl)+ complexes appear to be favorable for charge transfer. Finally, observations of Mg–Cl dissociation at the Mg surface at very low THF coordinations (0 and 1) suggest that deleterious Cl incorporation in the anode may occur upon plating. In the stripping process, this is beneficial by further facilitating the Mg removal reaction.