Abstract
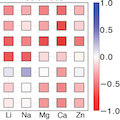
A thermodynamic analysis of the driving forces is presented for intercalation and conversion reactions in battery cathodes across a range of possible working ion, transition metal, and anion chemistries. Using this body of results, the importance of polymorph selection as well as chemical composition on the ability of a host cathode to support intercalation reactions is analyzed. It is found that the accessibility of high energy charged polymorphs in oxides generally leads to larger intercalation voltages favoring intercalation reactions, whereas sulfides and selenides tend to favor conversion reactions. Furthermore, it is observed that Cr‐containing cathodes favor intercalation more strongly than those with other transition metals. Finally, it is concluded that two‐electron reduction of transition metals (as is possible with the intercalation of a 2+ ion) will favor conversion reactions in the compositions studied.